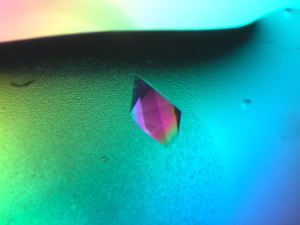
Description:

Summary
Improved growth of protein crystals using molecularly imprinted
polymers.
Scientists from the University of Surrey and Imperial College London have
come up with an invention
which uses hydrogel based molecularly imprinted polymers (HydroMIPs) for protein
crystallization, providing a higher yield of protein crystals over current
techniques. This method also works with protein structures that are difficult to
crystallise using other methods.
MIPs are polymers which are formed in the presence of a molecule
which once extracted leaves behind complamentary cavities. These allow highly
selective rebinding of the original molecule.
Obtaining useful crystals remains a problem in proteomics and
structural biology. Previously identified nucleants with desirable properties
for the task fall down because they have no specificity for individual proteins
however our novel technique takes this difficulty away as the MIPs are designed
specifically to attract a template protein.
Benefits
- MIPs act as
effective nucleants for the formation of large single crystals of otherwise
hard to crystallize proteins
- Improved
accuracy in screening experiments leading to successful hits missed by other
types of nucleant
Applications
- Protein
Crystallization
- Protein
purification/isolation
- Replacement
of biological antibodies in immunoassays
- Catalysis
- Biosensors
for medicine, food and the environment
Availability
Available for
licensing/developmental partnership
IP
status
Patent
Pending
Technical
In the paper "Protein crystallization facilitated by molecularly
imprinted polymers" (published in PNAS) seven proteins
were tested by imprinting them in MIPS. These were lysozyme, trypsin, catalase,
haemoglobin, intracellular xylanase IXT6-R217W, alphas crustacyanin and human
macrophage migration inhibitory factor.
All with the exception of catalase were able to induce the formation of
crystals of nine tested proteins. This included some non-cognate proteins with
similar molecular weight.
One of the crystals grown was that of HIV protein with a
diffraction of 4.2Å. Other techniques that have tried to crystallize this
complex have failed and have produced crystals with diffraction beyond
9Å.
When the experiments were repeated under the same conditions using
other known nucleants e.g. human hair, zeolites etc. Only lysozyme and trypsin
generated crystals and these were small multiple crystals compared to the large
single crystals produced using MIPs.
The hit rate index screens for certain proteins showed four of five
hits when the cognate MIPs were present and no hits when they were not. The
results show that in the presence of MIPS, 8 to 10% of the screening trials of
the target proteins produced hits that would.